How to Predict the Products of Alkene Reactions (Without Second-Guessing Yourself)
- ochemmasters
- Dec 11, 2024
- 6 min read
Updated: Nov 14, 2025
Alkene reactions are one of the first big “walls” students hit in organic chemistry. At first glance, it feels like there are a million reactions to memorize, each with its own rules. But here’s the truth: alkene addition reactions follow predictable patterns. In this post, I’ll show you how to confidently identify what gets added across the double bond — and also, where it ends up — so you can finally feel in control of these reactions.

Section 1: What is electrophilic addition to alkenes?
Organic chemistry often feels like a puzzle — and when it comes to alkenes, the trick is knowing what pieces get added and where they go.
In alkene addition reactions, the double bond acts as a reactive site (aka the Nucleophile). Specifically, the π bond (pi bond) between the two carbon atoms is broken and replaced with two new σ (sigma) bonds — one on each of those carbon atoms.
These reactions follow a consistent pattern:
→ 1 π bond is broken, and 2 σ bonds are formed.

The atoms or groups that get added are almost always represented as a pair, X and Y — for example:
In hydrohalogenation (HBr), X is H and Y is Br .

In halogenation (Br₂), X is Br and Y is another Br.

In hydroboration-oxidation (BH₃ + H₂O₂), X is H and Y is OH.

So while the process of addition is the same each time — breaking a pi bond and forming two new sigma bonds — the identity of X and Y, and how they are added, changes with each reaction.
That’s why the key to predicting the products of alkene additions quickly and effectively is to be able to answer three guiding questions — what, where, and how — about X and Y. We’ll break these down in the next section.
Essentially, if you can memorize the answers to these three questions for each reaction type, you’ll be able to accurately predict the major product MOST of the time — even when molecules look unfamiliar.
Section 2: The 3 Guiding Questions That Unlock Every Alkene Reaction
If you want to master addition reactions, stop trying to memorize every single mechanism — and instead, focus on learning to ask the right questions about each reagent.
Every alkene reaction boils down to this:
What is being added?
Where is it being added?
How is it being added?
Let’s break each one down:
1. What are X and Y? (What's being added?)
This is the easiest question and the first thing to identify. In every reaction, a pair of atoms or groups is added across the double bond — and they always come from the reagents.
In HBr, X = H and Y = Br
In Br₂, X = Br and Y = Br
In BH₃/THF + H₂O₂/NaOH, X = H and Y = OH
In H₂ + Pd, X = H and Y = H
By memorizing what each reagent adds, you’re already one-third of the way to predicting the product.
2. Where do X and Y add? (Regioselectivity)
Now that you know what’s being added, the next step is to figure out where each group lands — this is called regioselectivity.
Use this general rule of thumb:
If the reaction follows Markovnikov’s Rule, the more electronegative group (like OH or Br) goes on the more substituted carbon.
If it’s anti-Markovnikov, the electronegative group goes on the less substituted carbon.
Let’s compare:
HBr → Markovnikov (Br goes on the more substituted carbon)

BH₃/THF + H₂O₂/NaOH → Anti-Markovnikov (OH goes on the less substituted carbon)

Recognizing the regioselectivity tells you where X and Y end up on the molecule.
3. How are X and Y added in space? (Stereochemistry)
This is the step most students overlook — but it can make or break your answer.
Addition reactions often show stereoselectivity, meaning atoms are added to specific faces of the molecule. Something that often confuses students is that we ONLY need to show stereochemistry when chiral centers form. So in the following examples, you will see that some atoms will be drawn planar while others will have wedges/dashes.
There are 2 types of stereoselectivity:
Syn addition = both X and Y add to the same face
Anti addition = X and Y add to opposite faces
If there’s no stereoselectivity, then multiple stereoisomers (such as enantiomers or diastereomers) may form
⚠️ Important Caveat:
You only need to draw stereochemistry when a chiral center is formed. So even if a reaction is described as syn or anti, not all atoms will be drawn with wedges or dashes unless chirality is involved. Some atoms will remain planar in the product and H atoms do not need to be drawn out in the final product— that’s perfectly correct. See the examples below.
Examples:
Br₂ → anti addition

BH₃/THF + H₂O₂/NaOH → syn addition


HBr (via carbocation) → no stereoselectivity (multiple stereoisomers may form)

🧪 Section 3: Applying the Framework — Your Product Prediction Guide
Now that you understand the three guiding questions — what’s added, where it’s added, and how it’s added — you’re ready to apply them to real reactions.
Instead of overwhelming you with a long list of reactions, I’ve created a visual guide that breaks down the most common alkene addition reactions. In the guide, you'll find:
What’s being added (X and Y)
Whether the reaction follows Markovnikov or anti-Markovnikov regioselectivity
Whether the addition is syn, anti, or non-stereoselective
Visuals of the reactants, predicted products, and important features of each reaction
👉 Click here to download the Addition Reaction Cheat Sheet
Whether you're reviewing for an upcoming quiz or trying to finally get all these reactions to click — this guide is your go-to tool for organizing and mastering alkene additions.
⚠️ Section 4: Where Students Go Wrong
The “What, Where, and How” framework is an incredibly powerful tool — and it can help you predict the products of most alkene addition reactions with ease. But like anything in organic chemistry, there are exceptions.
Let’s break down some of the most common pitfalls students encounter:
1. Assuming the Framework Works 100% of the Time
While most addition reactions follow predictable patterns, reactions involving carbocation intermediates (like HBr or hydration via H₃O⁺) can sometimes produce unexpected products. This happens when carbocation rearrangements occur — such as hydride shifts or methyl shifts — leading to more stable intermediates and products you may not have anticipated.

🧠 Solution:
For these specific reactions, always take a moment to draw out the mechanism before predicting products. Don’t rely solely on the “What, Where, How” framework — let the mechanism guide you.
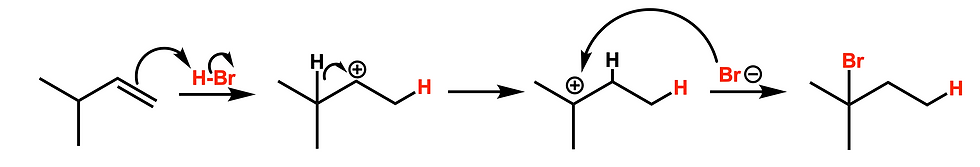
2. Confusing Chiral vs. Meso Products
One of the most misunderstood parts of stereochemistry in addition reactions is knowing when multiple stereoisomers form — and when they don’t.
Here’s the rule:
If the product has a new chiral center, then multiple stereoisomers (like enantiomers or diastereomers) may form.

If the product is meso, even if it has chiral centers, only one product forms due to internal symmetry.

This is especially important in halogenation reactions (e.g., Br₂ addition) or hydrogenation (i.e. H₂ addition), where students often expect enantiomers but the product is meso if the starting alkene is symmetric. See the example below:
🧠 Solution:
Always check the symmetry of the molecule and determine if a chiral center is created before deciding how many stereoisomers to draw.
3. Studying Harder, Not Smarter
Too many students spend hours reviewing textbook problems or low-level homework assignments without realizing these don’t reflect the difficulty or style of their professor’s exam.

versus

🧠 Solution:
Focus your energy on targeted practice:
Start with foundational problems
Then move toward increasingly complex and multi-step questions
Prioritize practice exams, worksheets, or materials created by your professor or others at your school
4. Overlooking Reagent Variations
Some professors like to mix things up. A small change — like switching H₂O to ROH in an acid-catalyzed hydration — can change the product from an alcohol to an ether.

Another common variation is when H₂SO₄/H₂O is used in place of H₃O⁺, or vice versa. These setups represent the same reaction mechanism — acid-catalyzed hydration — but are written differently depending on the professor or textbook.

🧠 Solution:
Always pay close attention to how reagents are written, and be ready to translate between different forms of the same reaction. Focus on what’s being added (X and Y) and under what conditions. Practice identifying patterns, not just memorizing reagent names.
5. Not Learning the Mechanisms (When Required)
Not every professor emphasizes mechanisms — but some require you to draw every arrow. If that’s the case, you’ll want to know the major ones inside and out.
The most commonly tested mechanisms for alkene addition include:
Hydrohalogenation (HX)
Acid-Catalyzed Hydration (H₂SO₄/H₂O or H₃O⁺)
Halogenation (X₂)
Halohydrin Formation (X₂, H₂O)
Oxymercuration-Demercuration (Hg(OAc)₂ H₂O followed by NaBH₄)
🧠 Solution:
Ask your professor directly:
“Which reaction mechanisms are we expected to know for the exam?”
When in doubt, learning the mechanisms not only helps you answer mechanism-based questions but also deepens your understanding of how and why the products form the way they do.
Mastering addition reactions takes more than memorizing mechanisms — it’s about learning to recognize patterns and predict reactivity with confidence. The more you practice breaking problems into steps, identifying intermediates, and applying reaction principles, the more intuitive these questions become.
If you found this guide helpful, take the next step — download my free Addition Reactions Practice Sheet or join my Orgo Study Club to test what you’ve learned and keep strengthening your skills!




Comments